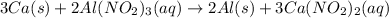Answer : The balanced chemical reaction will be:
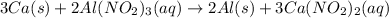
Explanation :
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
When solid calcium metal reacts with aqueous aluminum nitrate then it gives aqueous calcium nitrate and solid aluminium.
The balanced chemical reaction will be: