Answer:
2.5 moles of N₂ and 7.5 moles of H₂ entered the reaction
Step-by-step explanation:
In reaction:
N₂(g) + 3 H₂(g) → 2 NH₃(g)
You can see that the stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) requires the following amounts of reagents and are produced:
- N₂: 1 mole
- H₂: 3 moles
- NH₃: 2 moles
The following three rules can apply:
- If 2 moles of NH₃ are produced from 1 mole of N₂ by stoichiometry of the reaction, 5 moles of NH₃ from how many moles of N₂ are produced?
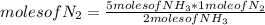
moles of N₂= 2.5
- If 2 moles of NH₃ are produced from 3 moles of H₂ by stoichiometry of the reaction, 5 moles of NH₃ from how many moles of H₂ are produced?
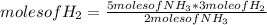
moles of H₂= 7.5
2.5 moles of N₂ and 7.5 moles of H₂ entered the reaction