Answer:
The answer is "2.5 mole"
Step-by-step explanation:
The reaction for producing
 can be defined as follows:
can be defined as follows:
Reaction:
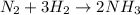
According to the above reaction, to produce 2 moles of
 we need 1 mole of nitrogen:
we need 1 mole of nitrogen:
So, according to the question to produce 5.0 mole
 the required
the required
 :
:
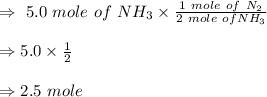
To produce 5.0 mole
 we need 2.5 mole
we need 2.5 mole
