The question is incomplete. Her eis the complete question.
Steam reforming methane (CH4) produces "synthesis gas", a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 125L tank with 20 mol of methane gas and 10 mol of water vapor at 38°C. He then raises the temperature, and when the mixture has come to equilibrium measures the amount of gas hydrogen to be 18 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to significant digits.
Answer:
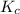 = 2.
= 2.
Explanation: The reaction for steam reforming methane is:
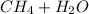 ⇒
⇒
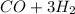
To calculate the concentration equilibrium constant, first calculate the molarity (
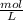 ) of each molecule of the reaction.
) of each molecule of the reaction.
At 38°C: At the initial temperature, there no products yet
Molarity of CH4:
CH4 =
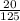 = 0.16M
= 0.16M
Molarity of H20:
H2O =
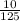 = 0.08M
= 0.08M
At final temperature:
Molarity of H2:
H2 =
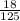 = 0.144M
= 0.144M
According to the chemical reaction, the combination of 1 mol of each reagents produces 1 mol of CO and 3 mols of H2, so, for the products, the ratio is 1:3.
Molarity of CO:
CO =
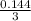 = 0.048M
= 0.048M
For the reagents, the proportion is 1:1, but they had an initial concentration, so, when in equilibrium, the concentration will be:
Molarity of CH4:
CH4 = 0.16 - 0.048 = 0.112M
Molarity of H2O:
H20 = 0.08 - 0.048 = 0.032M
The equilibrium constant is given by:
![K_(c) = ([CO][H_(2)]^(3) )/([CH_(4)][H_(2)O ] )](https://img.qammunity.org/2021/formulas/chemistry/college/haiwedrm9m05n1qx5fld93las7aaj95ibw.png)
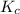 =
=
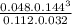
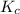 = 2.
= 2.
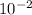
The concentration equilibrium constant for the process is
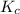 = 2.
= 2.
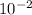 .
.