Answer:
2.56 grams of H₂S is needed to produce 18.00g of PbS if the H2S is reacted with an excess (unlimited) supply of Pb(CH₃COO)₂
Step-by-step explanation:
The balanced reaction is:
Pb(CH₃COO)₂ + H₂S → 2 CH₃COOH + PbS
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) they react and produce:
- Pb(CH₃COO)₂: 1 mole
- H₂S: 1 mole
- CH₃COOH: 2 moles
- PbS: 1 mole
In this case, to know how many grams of H₂S are needed to produce 18.00 g of PbS, it is first necessary to know the molar mass of the compounds H₂S and PbS and then to know how much it reacts by stoichiometry. Being:
- H: 1 g/mole
- S: 32 g/mole
- Pb: 207 g/mole
The molar mass of the compounds are:
- H₂S: 2* 1 g/mole + 32 g/mole= 34 g/mole
- PbS: 207 g/mole + 32 g/mole= 239 g/mole
So, by stoichiometry they react and are produced:
- H₂S: 1 mole* 34 g/mole= 34 g
- PbS: 1 mole* 239 g/mole= 239 g
Then the following rule of three can be applied: if 239 grams of PbS are produced by stoichiometry from 34 grams of H₂S, 18 grams of PbS from how much mass of H₂S is produced?
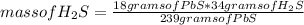
mass of H₂S= 2.56 grams
2.56 grams of H₂S is needed to produce 18.00g of PbS if the H2S is reacted with an excess (unlimited) supply of Pb(CH₃COO)₂