Answer: D=8900g/L
Step-by-step explanation:
Density is mass/volume. Mass in in grams and volume is in liters.
1 cm³=1 mL
With this conversion, we can convert cm³ to L.
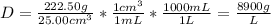
When we convert, we cancel out cm³ and mL to get L alone. The density is 8900 g/L.