Answer:
*
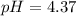
* The solution is acidic since the pH is below 7.
Step-by-step explanation:
Hello,
In this case, we can mathematically define the pH by:
![pH=-log([H_3O^+])](https://img.qammunity.org/2021/formulas/chemistry/college/etqosn0h9okk7kyy727j9bh8auakr0as2f.png)
Thus, for the given hydronium concentration we simply compute the pH:
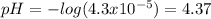
Thereby, we conclude the solution is acidic due to the fact that the pH is below 7 which is the neutral point and above it the solutions are basic.
Regards.