Answer:
10.41 mL of concentrated 12 M HCl (aq) is needed to prepare a 250mL of 0.5 M HCl solution
Step-by-step explanation:
Dilution is the procedure to prepare a less concentrated solution from a more concentrated one. The amount of solute does not vary, but the volume of the solvent varies. More solvent is added and the concentration of the solute decreases, as the volume of the solution increases.
In this case it is fulfilled:
Vi * Ci = Vf * Cf
Vi = initial volume
Ci = initial concentration
Vf = final volume
Cf = final concentration
Being:
- Vi=?
- Ci= 12 M
- Vf= 250 mL= 0.250 L (being 1 L= 1000 mL)
- Cf= 0.5 M
Replacing:
Vi*12 M= 0.250 L* 0.5 M
Solving:
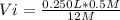
Vi= 0.01041 L= 10.41 mL
10.41 mL of concentrated 12 M HCl (aq) is needed to prepare a 250mL of 0.5 M HCl solution