Answer:
A. K = 0.546 eV
B. cooper and iron will not emit electrons
Step-by-step explanation:
A. This is a problem about photoelectric effect. Then you have the following equation:
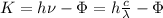 (1)
(1)
K: kinetic energy of the ejected electron
Ф: Work function of the metal = 2.48eV
h: Planck constant = 4.136*10^{-15} eV.s
λ: wavelength of light = 410nm - 750nm
c: speed of light = 3*10^8 m/s
As you can see in the equation (1), higher the wavelength, lower the kinetic energy. Then, the maximum kinetic energy is obtained with the lower wavelength (410nm). Thus, you replace the values of all variables :

B. First you calculate the energy of the photon with wavelengths of 410nm and 750nm
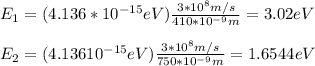
You compare the energies E1 and E2 with the work functions of the metals and you can conclude:
sodium = 2.3eV < E1
cesium = 2.1 eV < E1
cooper = 4.7eV > E1 (this metal will not emit electrons)
iron = 4.5eV > E1 (this metal will not emit electrons)