Answer:
The reaction is not in equilibrium and must move in a backward manner i.e towards the reactant so that it will attain equilibrium
Step-by-step explanation:
The complete question is as follows;
Consider the following reaction where Kc = 2.90×10-2 at 1150 K: 2 SO3 (g) 2 SO2 (g) + O2 (g) A reaction mixture was found to contain 4.71×10-2 moles of SO3 (g), 5.00×10-2 moles of SO2 (g), and 4.53×10-2 moles of O2 (g), in a 1.00 liter container.
Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals . The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.
Solution
The first thing to do here is to calculate the pressure of each of the gases. This would be useful in the equilibrium calculations. We calculate this by dividing the respective number of moles by the volume of the container.
Now, since the volume of the container is 1L, then the number of moles will be equal to the pressure of the gaseous substances, although units will be different.
So, [SO3] = 4.71 * 10^-2 mol/L
[SO2] = 5.00 * 10^-2 mol/L
[O2] = 4.53 * 10^-2 mol/L
The equation of the reaction is as follows;
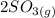 ⇆
⇆
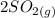 +
+
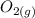
We proceed to calculate the reaction quotient Qc
Mathematically Qc for this reaction = [
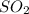 ]^2 × [
]^2 × [
 ]/ [
]/ [
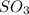 ]^2
]^2
Qc = {(5 * 10^-2)^2 * (4.53 * 10^-2)}/ (4.71 * 10^-2)^2 = 5.11 × 10^-2 mol/L
Now, we are given that the value of Kc = 2.9 * 10^-2 which is less than Qc
Since Kc < Qc, the backward reaction is favored.
Now to the question;
The reaction is not in equilibrium and must move in a backward manner i.e towards the reactant so that it will attain equilibrium