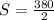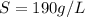Complete Question
A chemistry student weighs out 0.950 kg of an unknown solid compound and adds it to 2.00 L of distilled water at . After minutes of stirring, only some of the has dissolved. The student drains off the solution, then washes, dries and weighs the that did not dissolve. It weighs 0.570 kg.
Required:
a. Using the information above, can you calculate the solubility of X?
b. If so, calculate it. Remember to use the correct significant digits and units. .
Answer:
a
Yes the solubility of X can be calculated this is because the solubility of a substance dissolved in a solution is the amount of that substance that is needed to saturate 1 unit volume of the solvent solution at that given temperature.
And from our question we see that substance X saturated the solvent and there is still remained undissolved substance X
b
The solubility of X is
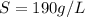
Step-by-step explanation:
From the question we are told that
The initial mass of the unknown solid is
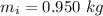
The mass of the undissolved substance is
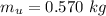
The volume of the solution is
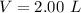
Yes the solubility of X can be calculated this is because the solubility of a substance dissolved in a solution is the amount of that substance that is needed to saturate 1 unit volume of the solvent solution at that given temperature.
And from our question we see that substance X saturated the solvent and there is still remained undissolved substance X
The mass of the substance that dissolved (
 ) is mathematically represented as
) is mathematically represented as
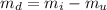
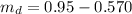
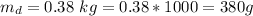
The solubility of this substance (X) is mathematically represented as
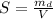
substituting values