Answer:
The time it'd take for the element to have 15 g of mass is approximately 68 min.
Explanation:
The radioactive decay of a substance is given by the following formula:
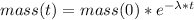
Since the element has a half life of 12 minutes, this means that after this time the mass of the element will be half of it was originally, therefore:
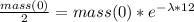
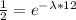
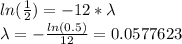
Therefore the mass of the element is given by:
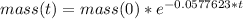
If the initial mass is 760 g and the final mass is 15 g, we have:
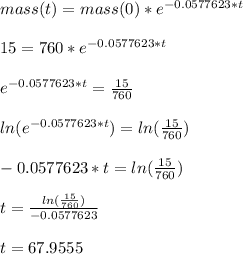
The time it'd take for the element to have 15 g of mass is approximately 68 min.