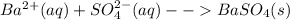Answer:
See the answer below.
Step-by-step explanation:
If an aqueous solution of two salts contains both Na2CO3 and Na2SO4, the following steps will prove the occurrence of both carbonate and sulphate ions:
1. Add a dilute acid (such as HCl) to the solution. The presence of carbonate ion will result in the release of carbon dioxide gas which will be shown by formation of effervescent bubbles. The gas can be proven to be carbon dioxide by channeling it into a lime water which usually turns milky with the presence of the gas.
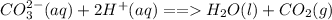
2. Add barium chloride to an acidified portion of the aqueous solution. The presence of sulphate ion will be indicated by the formation of white barium sulphate precipitate. Initial acidification is done to disperse off any carbonate ion that might be present in the solution and give a false-positive white precipitate result.