Answer:
mass of ethanolamine 61.2 g
Step-by-step explanation:
Data:
V (Volume) = 60 mL
ρ (Density)= 1.02
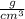 = 1.02
= 1.02
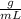
With the given data and using the density formula:
ρ =

It is possible to calculate the grams of ethanolamine that the student should weigh out. The mass in the formula is cleared and the data is replaced.
mass = ρ * volume
mass = 1.02
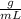 * 60 mL = 61.2 g of ethanolamine
* 60 mL = 61.2 g of ethanolamine