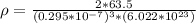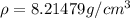Complete Question
The complete question is shown on the first uploaded image
Answer:
The density is
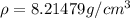
Step-by-step explanation:
From the question we are told that
the lattice constant is
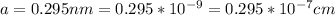
The density of Copper (
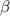 ) is mathematically represented as
) is mathematically represented as
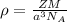
Where Z is the number of units in a unit cell and for BCC crystals Z =2
M is the molar mass of copper which same for Copper with a value of
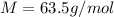
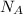 is the Avogadro's constant with a value of
is the Avogadro's constant with a value of
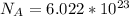
Substituting values