Answer:
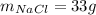
Step-by-step explanation:
Hello,
In this case, in order to compute the grams of sodium chloride starting by the molecules, the first step is to compute the moles contained in the given amount of molecules by using the Avogadro's number:
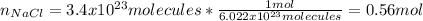
Then, by using the molar mass of sodium chloride (58.45 g/mol) we can directly compute the grams:
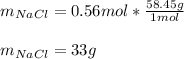
Regards.