Answer:
The concentration of the resulting solution in parts per million is 177.97
Step-by-step explanation:
Parts per million (ppm), is a unit of measure for concentration that refers to the number of units of the substance per million units of the set.
The concentration in parts per million expressed in mass / mass is calculated by dividing the mass of the solute (ms) by the mass of the solution (md, sum of the mass of the solute and the mass of the solvent), both expressed in the same unit and multiplied by 10⁶ (1 million).
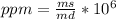
So, being:
- md: 0.089 grams of KI + 500 grams of H₂O= 500.089 grams
Replacing:
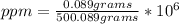
ppm= 177.97
The concentration of the resulting solution in parts per million is 177.97