Answer: 1.
 increases.
increases.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
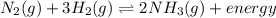
If the concentration of
 is increased , according to the Le-Chatlier's principle, the reaction has to shift to right or forward direction. In order to do that the concentration of products has to increase.
is increased , according to the Le-Chatlier's principle, the reaction has to shift to right or forward direction. In order to do that the concentration of products has to increase.
Thus the concentration of
 increases.
increases.