Answer:
0.032 M
Step-by-step explanation:
pH denotes the acidity or alkalinity of an aqueous solution. pH stands for potential hydrogen. The letter “p” denotes potential and the letter “H” denotes for hydrogen. A pH number varies from 0 to 14 depending upon on how acidic or alkaline a liquid is. Any aqueous solution that has pH above 7 is alkaline and any aqueous solution that has pH below 7 is acidic.
ph of phosphoric acid = 1.5
pH = -log[
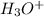 ]
]
1.5 = -log[
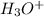 ]
]
-1.5 = log[
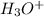 ]
]
![[H_3O^+]=10^(-1.5)](https://img.qammunity.org/2021/formulas/chemistry/high-school/totk8dof3v7z4lj7vwww9gzjm70azyajla.png) = 0.032 M
= 0.032 M