Answer:
Step-by-step explanation:
Half life of Nobelium-253 is 97 seconds . That means after every 97 seconds half of the Nobelium amount will be disintegrated .
Time taken in bringing the sample to laboratory = 291 seconds
291 second = 291 / 97 half life
n = 3
N =
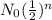
N₀ is original mass , N is mass after n number of half life.
N = 5 mg x
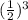
= .625 mg
Only 0.625 mg of Nobelium-253 will be left .