Answer:
The correct answer is 201.7 g
Step-by-step explanation:
Silver is a chemical element which symbol is Ag. From the Periodic Table, the molar mass of Ag is 100.86 g/mol. Also, we know that 1 mol of atoms is equal to 6,022 x 10²³atoms (Avogadro's number). So, we have the following relations (convertion factors):
1 mol Ag atoms = 6,022 x 10²³ atoms
1 mol Ag = 100.86 g
We use this factors and we calculate the grams og Ag as follows:
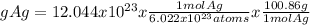 = 201.7 g
= 201.7 g