Answer:
1335.12 mL of H2O
Step-by-step explanation:
To calculate the mililiters of water that the solution needs, it is necessary to know that the volume of the solution is equal to the volume of the solute (NaOH) plus the volume of the solvent (H2O).
From the molarity formula we can first calculate the volume of the solution:
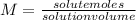
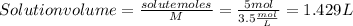
The volume of the solution as we said previously is:
Solution volume = solute volume + solvent volume
To determine the volume of the solute we first obtain the grams of NaOH through the molecular weight formula:
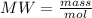
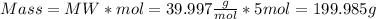
Now with the density of NaOH the milliliters of solute can be determined:
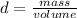
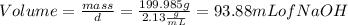
Having the volume of the solution and the volume of the solute, the volume of the solvent H2O can be calculated:
Solvent volume = solution volume - solute volume
Solvent volume = 1429 mL - 93.88 mL = 1335.12 mL of H2O