Answer:
1.
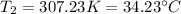
2.
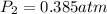
Step-by-step explanation:
Hello,
1. In this case, the standard pressure is 1 atm or 760 mmHg, therefore, using the Gay-Lussac's law we can compute the corresponding temperature considering the initial 50.0 °C in absolute Kelvins:
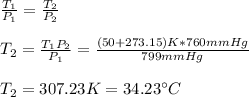
2. As well as in the previous case, we now compute the pressure at 273 K which is the standard temperature:
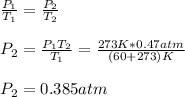
Best regards.