Answer:
13.14 moles of oxygen would be consumed if 460 g of water were produced
Step-by-step explanation:
First of all you must know the molar mass of the compounds that participate in the reaction. Being:
The molar mass of the reactants and products in the reaction are:
- H₂: 2* 1 g/mole= 2 g/mole
- O₂: 2* 16 g/mole= 32 g/mole
- H₂O: 2* 1 g/mole + 16 g/mole= 18 g/mole
Now, by stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), they react and are produced:
- H₂: 2 moles
- O₂: 1 mole
- H₂O: 2 moles
Then, stoichiometry reacts 2 moles * 18 g/mole = 36 g of H₂O.
Then you can apply the following rule of three: if 35 grams of H₂O are formed from 1 mole of O₂, 460 grams of H₂O from how many moles of O₂ are formed?
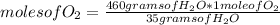
moles of O₂= 13.14
13.14 moles of oxygen would be consumed if 460 g of water were produced