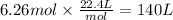Answer:
140 L
Step-by-step explanation:
Step 1: Write the balanced equation
2 H₂(g) + O₂(g) → 2 H₂O(g)
Step 2: Calculate the moles corresponding to 100 g of oxygen
The molar mass of oxygen is 32.00 g/mol.
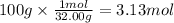
Step 3: Calculate the moles of water vapor formed
The molar ratio of oxygen to water vapor is 1:2.
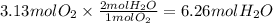
Step 4: Calculate the volume corresponding to 6.26 moles of water vapor
1 mole of any ideal gas under STP has a volume of 22.4 L.