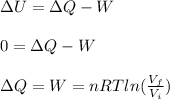Answer:
a) 0 J
b) W = nRTln(Vf/Vi)
c) ΔQ = nRTln(Vf/Vi)
d) ΔQ = W
Step-by-step explanation:
a) To find the change in the internal energy you use the 1st law of thermodynamics:
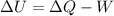
Q: heat transfer
W: work done by the gas
The gas is compressed isothermally, then, there is no change in the internal energy and you have
ΔU = 0 J
b) The work is done by the gas, not over the gas.
The work is given by the following formula:
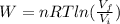
n: moles
R: ideal gas constant
T: constant temperature
Vf: final volume
Vi: initial volume
Vf < Vi, then W < 0 and the work is done on the gas
c) The gas has been compressed. Thus, its temperature increases and heat has been transferred to the gas.
The amount of heat is equal to the work done W
d)