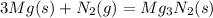Answer:
Step-by-step explanation:
The valance of magnesium atom is
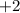
While the valence of nitrogen atom is
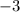
Let us first write the first half-reactions
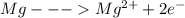
The second half reaction is
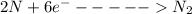
Adding the above two reactions and writing the final reaction, we get -
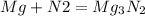
The balance equation is