Answer:
Double displacement chemical reaction.
Step-by-step explanation:
Hello,
In this case, for the given reaction we firstly balance it:
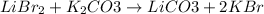
Then, since the lithium and potassium cations are being exchanged to each other from bromide and carbonate, we are talking about a double displacement chemical reaction.
Best regards.