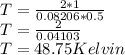Answer:
The temperature of the gas is 48.75 Kelvin.
Step-by-step explanation:
Using the ideal gas equation as shown
PV = nRT where;
P is the pressure of the gas in ATM
V is the volume of the gas
n is the number of moles
R is the ideal gas constant
T is the temperature in Kelvin
From the formula,
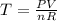
Given the following parameters V = 1litre, n = 0.5moles. pressure = 2ATM
R = 0.08206 atm L/molK
On substituting to get the temperature we have: