Answer:
242.5kPa
Step-by-step explanation:
According to one of the gas laws,
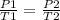
Given P1 = 240.0kPa, T1, 15.0°C, T2 = 18.0°C, P2 = ?
Substituting this values into the equation, we have;
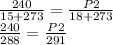
Cross multiplying we have;
288P2 = 240*291
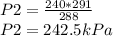
The new pressure is 242.5kPa