Answer:
 is the limiting reagent
is the limiting reagent
Step-by-step explanation:
To calculate the moles :
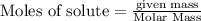
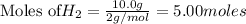
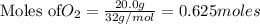
The balanced chemical reaction is :
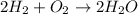
According to stoichiometry :
1 moles of
 require = 2 moles of
require = 2 moles of

Thus 0.625 moles of
 will require=
will require=
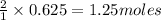 of
of

Thus
 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
 is the excess reagent as it is present more than the required amount.
is the excess reagent as it is present more than the required amount.