Answer:
Q = 114895 J
Step-by-step explanation:
To find the thermal energy gained by the ice you use the following formula:
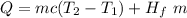
m: mass of the ice = 0.250kg
T2: final temperature = 22°C
T1: initial temperature = -8.0°C
Hf: heat of fusion of water = 3.34*10^5 J/kg
c: specific heat of water = 4186 J/kg
By replacing the values of the parameters you have:
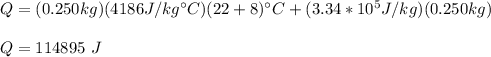
where you have considered that ice melts completely