Answer:
The correct answer to the following question will be Option E (1.0 x 10⁻³ M).
Step-by-step explanation:
The given values are:
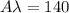
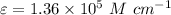
L = 1 \ cm
As we know,
⇒
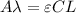
On putting the given values in the above formula, we get
⇒
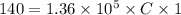
⇒
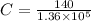
⇒
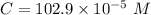
So that option E is the right answer.