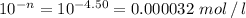Answer:
0.000032 mol/l
Step-by-step explanation:
A solution that has a higher concentration of hydrogen ions than water is known as an acidic solution and basic or alkaline solutions have a lower concentration of hydrogen ions than water.
The nucleus of a hydrogen atom separated from its electron is known as a hydrogen ion.
pH of an acidic solution (n) = 4.50
Hydroxide ion concentration of an acidic solution =