Answer:
Specific heat capacity of unknown liquid is given as,
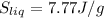 °
°
Step-by-step explanation:
Let the mass of unknown liquid is "m"
Now when unknown liquid is mixed with water then heat given by water is equal to the heat absorbed by unknown liquid
So we have
Msliq ( 50 - 20 ) = (240 - m) (4.2)(80-50)
Now again we did the same experiment but this time the mass of unknown liquid is "m - 20"
so we have
(m-20)Sliq(52-20)=(220-m)(4.2)(80-52)
now from above two equations
m(30)/(m-20)32 = (240-m)30/(220-m)/28
Now we have
30m * 28 (220 - m) = 32 ( m- 20) * 30 ( 240 - m)
so we have
m = 84.2g
Now we have
Sliq = 240-84.2/84.2 (4.2)
Sliq = 7.77J/g°C