Answer: Thus the volume of the container that the gas is in 2311 L
Step-by-step explanation:
According to ideal gas equation:

P = pressure of gas = 0.09 atm
V = Volume of gas = Volume of container = ?
n = number of moles = 7.7
R = gas constant =
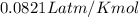
T =temperature =
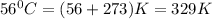
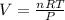
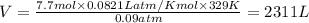
Thus the volume of the container that the gas is in 2311 L