Answer:
Both are equal
Step-by-step explanation:
Mass of starting material i.e Aluminium is
 grams
grams
The weight of oxygen is
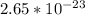
There are 4 atoms of aluminium and 3 atoms of oxygen
Thus, the total mass of starting material is
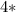 mass of one atom of aluminium
mass of one atom of aluminium
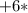 mass of one atom of oxygen
mass of one atom of oxygen
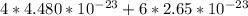
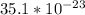 grams
grams
Mass of new substance is also equal to
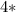 mass of one atom of aluminium
mass of one atom of aluminium
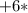 mass of one atom of oxygen
mass of one atom of oxygen
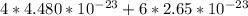
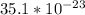 grams
grams