Answer:
There are 0.12269 Liters of Iron
Step-by-step explanation:
First, by means of the molecular weight equation, the grams of iron corresponding to 17.3 moles can be calculated:
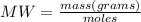
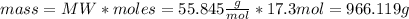
Then using the density equation the iron milliters are calculated:
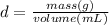
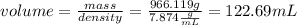
Milliliters to liters are converted:
122.69 mL = 0.12269 L of Iron.