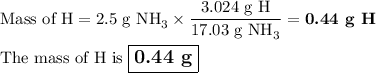Answer:

Explanation:
1. Calculate the molar mass of ammonia
1 N = 1 × 14.01 g = 14.01 g
3 H = 3 × 1.008 g = 3.024
TOTAL = 17.03 g
2. Calculate the mass of hydrogen
So, there are 3.024 g of H in 17.03 g of NH₃.
The conversion factor is either
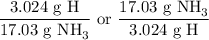
We want to find grams of H, so we choose the first one.