Answer: 2. Decomposition
Step-by-step explanation:
Synthesis reaction is a type of chemical reaction in which two reactants combine to give a single product.
Decomposition reaction is defined as the reaction where a single substance breaks down into two or more simpler substances.
lithium carbonate → lithium oxide + carbon dioxide
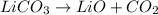
Single replacement reaction is a type of chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
A double replacement reaction is one in which exchange of ions take place.