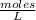Answer:
The molarity is 1.26
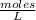
Step-by-step explanation:
Molarity is a unit of concentration that is based on the volume of a solution and represents the number of moles of solutes contained in a liter. The molarity of a solution is calculated by dividing the moles of the solute by the liters of the solution and is expressed in units (moles / liter).
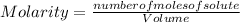
You must calculate the number of moles of CuSO₄. So, being:
- Cu: 63.54 g/mole
- S: 32 g/mole
- O: 16 g/mole
the molar mass of CuSO₄ is
CuSO₄=63.54 g/mole + 32 g/mole + 4* 16 g/mole= 159.54 g/mole
Then it is possible to apply the following rule of three: if 159.54 g of CuSO₄ are present in 1 mole, 10 g in how many moles are they?
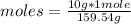
moles= 0.063 moles
Then:
- number of moles of solute= 0.063 moles
- Volume= 50 mL= 0.05 L (Being 1L=1000 mL)
Replacing in the definition of molarity:
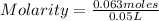
Molarity= 1.26
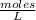
The molarity is 1.26