Answer:
5.9 kg
Step-by-step explanation:
We must work backwards from the second step to work out the mass of oxygen.
1. Second step
Mᵣ: 55.84
Fe₂O₃ + 3CO ⟶ 2Fe + 3CO₂
m/kg: 7.0
(a) Moles of Fe
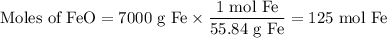
(b) Moles of CO
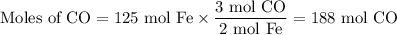
However, this is the theoretical yield.
The actual yield is 72. %.
We need more CO and Fe₂O₃ to get the theoretical yield of Fe.
(c) Percent yield
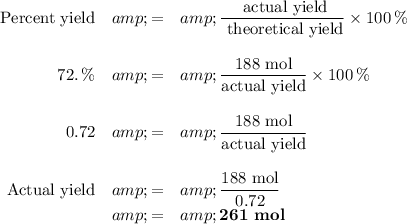
We must use 261 mol of CO to get 7.0 kg of Fe.
2. First step
Mᵣ: 32.00
2C + O₂ ⟶ 2CO
n/mol: 261
(a) Moles of O₂
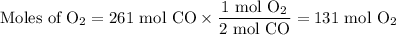
(b) Mass of O₂
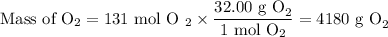
However, this is the theoretical yield.
The actual yield is 71. %.
We need more C and O₂ to get the theoretical yield of CO.
(c) Percent yield
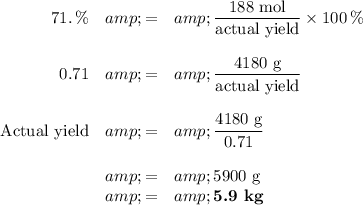
We need 5.9 kg of O₂ to produce 7.0 kg of Fe.