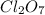Answer: 780 kcal are required to produce 12.00 mol of
Step-by-step explanation:
Endothermic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants. The total energy is absorbed in the form of heat and written along with reactants.
The balanced chemical reaction is:
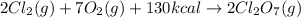
According to stoichiometry :
2 moles of
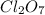 are produced by absorption of energy = 130 kcal
are produced by absorption of energy = 130 kcal
Thus 12.00 moles of
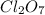 are produced by absorption of energy =
are produced by absorption of energy =
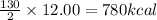
Thus 780 kcal are required to produce 12.00 mol of