Answer:
- Neutral solutions: concentration of hydronium equals the concentration of hydroxide.
- Acid solutions: concentration of hydronium is greater than the concentration of hydroxide.
- Basic solutions concentration of hydronium is lower than the concentration of hydroxide.
Step-by-step explanation:
Hello,
It is widely known that the pH of water is 7, therefore the pOH of water is also 7 based on:
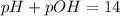
In such a way, we can compute the concentration of hydronium and hydroxide ions as shown below:
![pH=-log([H^+])\\](https://img.qammunity.org/2021/formulas/chemistry/college/av02t5rd5ylg8m6mzai95k32ef3v96wtlu.png)
![[H^+]=10^(-pH)=10^(-7)=1x10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/college/m14mbjjfsrc3naoyvmatji64wccueviigg.png)
![pOH=-log([OH^-])](https://img.qammunity.org/2021/formulas/chemistry/college/os6i8jhsq2yoevim3ewlj1jvyoxoba6pi1.png)
![[OH^-]=10^(-pOH)=10^(-7)=1x10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/college/p4a7wm58ni5xagvlsa0b066w3184jf5wd5.png)
Thus, we notice that the relationship between the concentration of the hydronium is equal for water or neutral solutions. Moreover, if we talk about acid solutions, pH<OH therefore the concentration of hydronium is greater than the concentration of hydroxide. On the other hand if we talk about basic solutions, pH>OH therefore the concentration of hydronium is lower than the concentration of hydroxide.
Best regards.