Answer:
T'=92.70°C
Step-by-step explanation:
To find the temperature of the gas you use the equation for ideal gases:
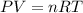
V: volume = 3000cm^3 = 3L
P: pressure = 1250mmHg; 1 mmHg = 0.001315 atm
n: number of moles
R: ideal gas constant = 0.082 atm.L/mol.K
T: temperature = 27°C = 300.15K
For the given values you firs calculate the number n of moles:
![n=(PV)/(RT)=((1520[0.001315atm])(3L))/((0.082(atm.L)/(mol.K))(300.15K))=0.200moles](https://img.qammunity.org/2021/formulas/physics/college/roj4px9aqpo8ceu3bkx1hqhxybvto50txl.png)
this values of moles must conserve when the other parameter change. Hence, you have V'=2L and P'=3atm. The new temperature is given by:
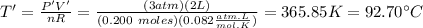
hence, T'=92.70°C