Answer:
![Ksp=[Ag^+]^2[(CO_3)^(-2)]](https://img.qammunity.org/2021/formulas/chemistry/college/r0pk3jv3vpg6seq1fp0aj3mj1mwpw4ikix.png)
Step-by-step explanation:
Hello,
In this case, we should start by writing the equilibrium dissociation of silver carbonate as:
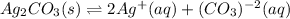
In such a way, by means of the law of mass action for heterogeneous equilibrium (as silver carbonate is solid), we represent the solubility product expression as shown below:
![Ksp=[Ag^+]^2[(CO_3)^(-2)]](https://img.qammunity.org/2021/formulas/chemistry/college/r0pk3jv3vpg6seq1fp0aj3mj1mwpw4ikix.png)
Best regards.