Answer:
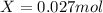
Step-by-step explanation:
Hello,
In this case, we need to apply a mole mass relationship via the molar mass of the magnesium bromide (MgBr2). For it, we first compute its molar mass as shown below:
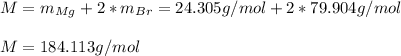
Hence, we can understand that 1 mole of magnesium bromide contains 184.113 grams of mangnesium bromide, for that reason, for 5 grams of magnesium bromide we have the following moles:
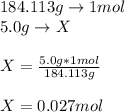
Best regards.