Answer:
At -13
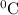 , the gas would occupy 1.30L at 210.0 kPa.
, the gas would occupy 1.30L at 210.0 kPa.
Step-by-step explanation:
Let's assume the gas behaves ideally.
As amount of gas remains constant in both state therefore in accordance with combined gas law for an ideal gas-
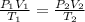
where
 and
and
 are initial and final pressure respectively.
are initial and final pressure respectively.
 and
and
 are initial and final volume respectively.
are initial and final volume respectively.
 and
and
 are initial and final temperature in kelvin scale respectively.
are initial and final temperature in kelvin scale respectively.
Here
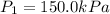 ,
,
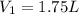 ,
,
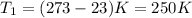 ,
,
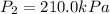 and
and
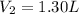
Hence
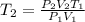
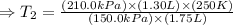
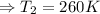
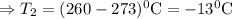
So at -13
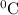 , the gas would occupy 1.30L at 210.0 kPa.
, the gas would occupy 1.30L at 210.0 kPa.