Answer:
pH
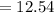
OH- concentration 28.84
Step-by-step explanation:
KOH dissociates into K+ and OH-. The ratio of K+ and OH- ion is 1:1
In any aqueous solution, the H3O+ and OH - must satisfy the following condition -
![[ H_3O^+] [OH^-] = k_w](https://img.qammunity.org/2021/formulas/chemistry/high-school/y40zv5kamt842lb26xdrttg8sm5qwvfvo4.png)
![[ H_3O^+] = (k_w)/( [OH^-])](https://img.qammunity.org/2021/formulas/chemistry/high-school/65gyew03g7dco6rs08xykm9qiheoaoqstc.png)
![[H_3O^+] = \frac{1 * 10^(-14)}{3.5 *10^(-2)[H_3O^+] = 2.857 * 10^(-13)]() M
M
pH =
![- log [ H_3O^+]\\- log [2.857 * 10^(-13)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/aizi8st9za2ttvuqrbw0tlneajytm5nccc.png)
pH
![= - [-12.54]\\= 12.54](https://img.qammunity.org/2021/formulas/chemistry/high-school/s21sw8rfpr3pcibp6n0ehi0ey25r7tqzgi.png)
pOH
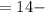 pH
pH
pOH
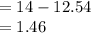
OH concentration
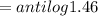
OH- concentration 28.84