Answer:
219.75g
Step-by-step explanation:
The balanced equation for this reaction is:
Pb + H2SO4 → PbSO4 + H2
mole ratio of lead to lead (II) sulfate is 1 : 1
1 mole of Pb = 207g
?? moles of Pb - 150g
⇒
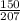 = 0.7246 moles
= 0.7246 moles
∴ mass of PbSO4 produced =
303.26 × 0.7246 = 219.75g